Most people assume that when a pharmaceutical company gets a patent on a new drug, they get 20 years to sell it without competition. That’s not true. In reality, most drugs have only 10 to 15 years of actual market exclusivity - and sometimes even less. The reason? By the time a drug hits the shelves, half the patent clock has already run out.
The 20-Year Myth
Patents are granted for 20 years from the date of filing. That’s the law. But here’s the catch: that clock starts ticking the moment scientists file the first patent application - often years before the drug even enters human trials. Most drugs take 8 to 12 years to get from the lab to the pharmacy. That means by the time the FDA approves the drug for sale, only 8 to 12 years of patent life remain. And that’s before you factor in manufacturing delays, pricing negotiations, or insurance coverage hurdles.Take a typical new cancer drug. Researchers file the first patent in 2010. Clinical trials start in 2012. Phase 3 trials wrap up in 2018. The FDA takes another 18 months to review it. Approval comes in 2020. That’s 10 years gone. The company now has just 10 years left on its patent - not 20. And if the drug is a biologic? That timeline stretches even longer, sometimes eating up 12 years before approval.
The Hatch-Waxman Act: A Compromise That Didn’t Go Far Enough
In 1984, Congress passed the Hatch-Waxman Act to fix this imbalance. The idea was simple: if it takes so long to get a drug approved, give the company back some of that lost time. The law allowed for Patent Term Extension (PTE) - up to five extra years of protection - to make up for regulatory delays.But there’s a hard cap: no matter how long the approval process took, the total market exclusivity can’t exceed 14 years from the date of FDA approval. That means if a drug got approved in 2015 and already had 11 years of patent life left, it could only get 3 more years of extension - not the full five. If it had only 6 years left, it could get the full five. But if it had 12 years left? No extension at all.
This system was designed to give companies just enough time to recoup their $2.6 billion investment (in 2013 dollars) - not to create decades-long monopolies. But reality didn’t follow the plan.
Secondary Patents: The Real Game-Changer
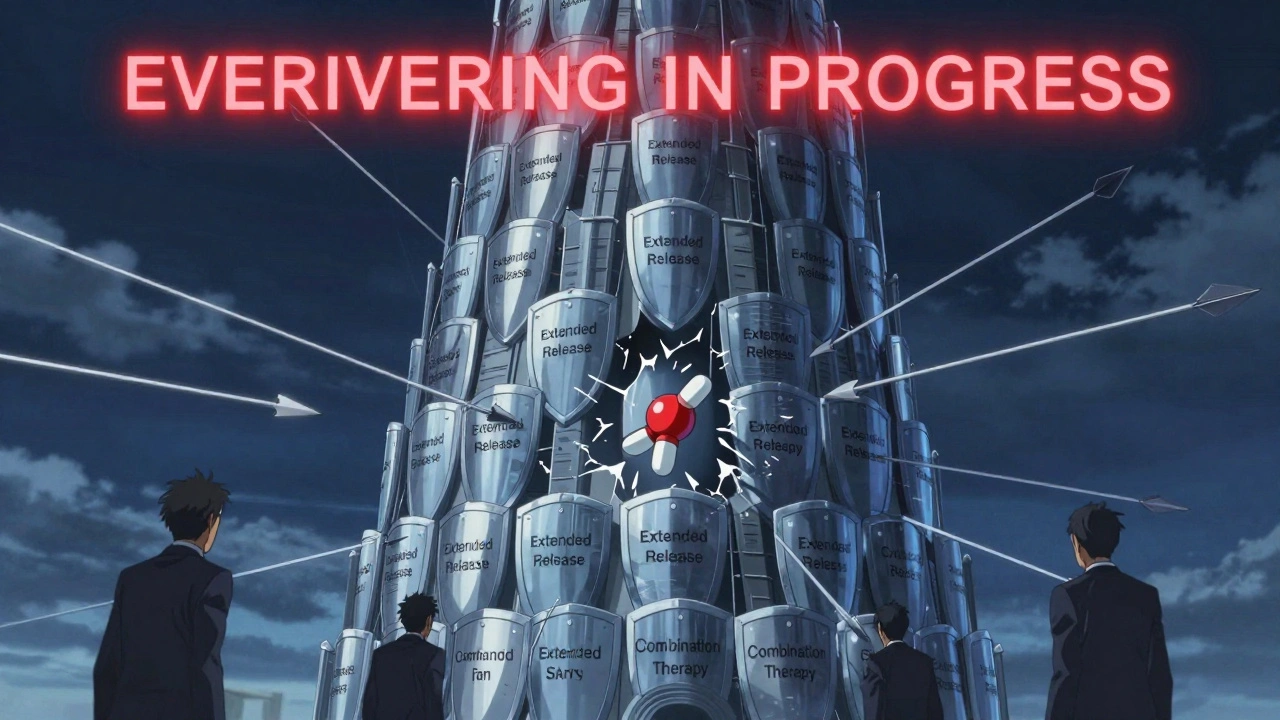
Here’s where things get complicated. Companies don’t rely on just one patent. They file dozens. These are called secondary patents - and they’re not about the active ingredient. They’re about how the drug is made, how it’s taken, or what it’s used for.Examples:
- A new extended-release version of a pill (patent on the coating)
- A different salt form of the same molecule (patent on crystallization)
- A combination with another drug (patent on the dosing schedule)
- A new use for an old drug (patent on treating depression instead of anxiety)
According to a 2023 study by the R Street Institute, blockbuster drugs average 20 to 30 patents each. And here’s the kicker: drugs with higher sales are 37% more likely to get these secondary patents. That’s not innovation - that’s strategy. It’s called “evergreening.”
These patents don’t always hold up in court. But they don’t have to. All they need to do is delay generic entry. If a generic company files to sell a copy, the brand company sues. Under Hatch-Waxman, that triggers a 30-month automatic stay. The FDA can’t approve the generic until the court decides - even if the patent is weak.
Regulatory Exclusivities: The Hidden Clock
Beyond patents, there’s another layer of protection: regulatory exclusivity. These are separate from patents and don’t require litigation to enforce.- New Chemical Entity (NCE) Exclusivity: 5 years of market protection - no generics allowed, even if the patent expires.
- New Clinical Investigation Exclusivity: 3 years for new uses or formulations of existing drugs.
- Orphan Drug Exclusivity: 7 years for drugs treating rare diseases (under 200,000 patients in the U.S.).
- Pediatric Exclusivity: 6 months added to any existing patent or exclusivity period if the company tests the drug in children.
These aren’t loopholes - they’re legal tools. And they stack. A drug might have NCE exclusivity (5 years), pediatric exclusivity (6 months), and a patent extension (3 years). That’s 8.5 years of protected time before generics can even apply - and that’s before secondary patents kick in.
Global Differences: It’s Not Just the U.S.
The U.S. isn’t alone in this game. Other countries have their own rules:- Canada: Certificate of Supplementary Protection (CSP) gives up to 2 years of extra protection after patent expiry.
- Japan: Patent Term Extension (PTE) can add up to 5 years, similar to the U.S.
- European Union: Supplementary Protection Certificates (SPCs) extend protection by up to 5 years, with a possible 6-month pediatric extension.
But the U.S. remains the most aggressive in stacking protections. A 2017 Yale Law Review study found that 91% of drugs that got patent extensions kept their monopoly well past expiration - thanks to secondary patents and exclusivities.

The Economic Reality: Billions at Stake
When a drug’s exclusivity ends, prices drop - fast. Generic versions can cut the price by 80% to 90% in the first year. For a blockbuster drug doing $2 billion in annual sales, that’s $1.6 billion lost overnight.That’s why companies spend millions on lifecycle management. They don’t just wait for the patent to expire. They’re already developing the next version - a new pill, a new injection, a new combo - while the old one is still on the market. The goal? To shift patients over before the generics arrive.
By 2025, over $250 billion in global drug sales are expected to face patent expiration. That’s not just a business problem - it’s a public health one. If generics can’t enter quickly, patients pay more. If they enter too fast, innovation slows.
Why This Matters to You
You might not care about patent law. But you care about how much your prescription costs. If a drug you take has a short effective patent life, you’ll see generics sooner - and pay less. If it’s protected by a thicket of secondary patents, you might be stuck paying full price for years longer than you should.And if you’re a patient with a rare disease? You might benefit from orphan drug exclusivity - but only if the company actually develops the drug. Some companies file for orphan status just to lock in 7 years of exclusivity - then charge $500,000 per year for a pill that barely works.
The system was built to balance innovation and access. But today, it leans heavily toward the latter - at the expense of patients and payers. The 20-year patent sounds like a long time. But in pharma, it’s just the starting line.
What is the average effective patent life for a new drug in the U.S.?
The average effective patent life - meaning the time a drug has exclusive market rights after FDA approval - is about 10 to 15 years. This includes both patent protection and regulatory exclusivities. Most of the original 20-year patent term is consumed during clinical trials and FDA review, leaving only about 7 to 13 years of actual exclusivity after approval.
Can a drug’s patent be extended beyond 20 years?
Yes, but only partially. The Hatch-Waxman Act allows for a Patent Term Extension (PTE) of up to five years to compensate for regulatory delays. However, the total exclusivity period - including the original patent and any extension - cannot exceed 14 years from the date of FDA approval. So while the patent number might show 25 years, the legal right to block generics is capped at 14 years after approval.
What’s the difference between a patent and regulatory exclusivity?
A patent protects the invention - the chemical structure or method of use - and is issued by the U.S. Patent Office. Regulatory exclusivity is granted by the FDA and protects the drug from generic competition based on its approval status, regardless of patents. For example, a drug can have 5 years of New Chemical Entity exclusivity even if no patent exists. Exclusivity doesn’t require lawsuits to enforce - it’s automatic.
Why do some drugs have 30+ patents?
Companies file multiple secondary patents on minor changes - like new pill coatings, different dosages, or combinations with other drugs. These aren’t always groundbreaking innovations. They’re legal tools to delay generic entry. A single blockbuster drug might have patents on its formulation, manufacturing process, method of use, and even packaging. Together, they create a “patent thicket” that makes it hard and expensive for generics to challenge them all.
Do generic drugs always come right after a patent expires?
Not always. Even after a patent expires, generic manufacturers must wait out any remaining regulatory exclusivity (like 5-year NCE protection). Plus, if the brand company sues the generic maker within 45 days of their application, the FDA is legally blocked from approving the generic for up to 30 months - even if the patent is weak or invalid. Many generics never make it to market because of these delays.
Is the current patent system working as intended?
Not really. The Hatch-Waxman Act was designed to balance innovation with affordable access, expecting most drugs to get 10-14 years of exclusivity after approval. But today, companies routinely extend monopolies far beyond that using secondary patents, evergreening, and strategic litigation. Studies show 91% of drugs with patent extensions maintain market control past expiration. The system now favors prolonged profits over timely generic entry - which drives up drug prices for everyone.
What Comes Next?
The pressure is building. Lawmakers are starting to question whether secondary patents should be allowed at all - especially for drugs that don’t offer real clinical improvements. Some proposals would limit patent extensions to only the original active ingredient, or require proof of significant benefit before granting new patents.Meanwhile, generic manufacturers are getting smarter. They’re pooling resources to challenge weak patents in court. Some are even developing biosimilars before the original drug’s patent expires - betting that the courts will rule in their favor.
For patients, the takeaway is simple: if you’re paying full price for a drug that’s been on the market for more than a decade, ask why. Chances are, it’s not because of innovation. It’s because of a legal system designed to protect profits - not patients.

Courtney Blake
December 11, 2025 AT 01:29So let me get this straight - we’re telling pharma companies they can’t monopolize drugs for 20 years, but they still somehow squeeze out 15? That’s not a system, that’s a rigged casino. And don’t even get me started on the ‘secondary patents’ - it’s like they’re playing Jenga with the law and calling it innovation. We’re paying $10,000 for a pill that’s been around since 2010 because some lawyer figured out how to patent the color of the capsule. This isn’t capitalism - it’s corporate feudalism.
Lisa Stringfellow
December 12, 2025 AT 23:37Ugh. I just read this and immediately thought about my dad’s diabetes meds. He’s been paying $600/month for the same pill for 12 years. I don’t care how many patents they file - if the active ingredient hasn’t changed, it shouldn’t cost more than $20. This whole system is just a tax on sick people. And the worst part? No one gets punished for it.
Michaux Hyatt
December 14, 2025 AT 06:52Really great breakdown - this is exactly the kind of info most people never see. The 20-year myth is everywhere, and it’s used to justify insane prices. But the real kicker? Regulatory exclusivities stack like Legos. NCE + pediatric + patent extension + secondary patents = 17+ years of monopoly. And none of it’s about improving patient outcomes. It’s about delaying the inevitable. The system wasn’t designed to fail - it was designed to be gamed. And guess who’s gaming it? The same companies that lobby Congress every year to keep it that way.
Also, bonus fact: the 30-month stay on generics? That’s not a legal loophole - it’s a corporate weapon. You don’t need to win the lawsuit. You just need to drag it out long enough for the generic company to run out of cash.
Raj Rsvpraj
December 14, 2025 AT 11:52Stephanie Maillet
December 15, 2025 AT 17:35It’s fascinating - and deeply tragic - how we’ve turned medicine into a legal chess game. The original intent of patents was to incentivize innovation, not to create infinite monopolies through semantic tweaks. A new coating? A different salt? These aren’t breakthroughs - they’re distractions. And yet, we’ve built an entire industry around exploiting legal technicalities instead of solving real medical problems. We’ve lost sight of the fact that drugs are meant to heal, not to generate quarterly earnings reports. At what point do we decide that human health shouldn’t be a derivative of intellectual property law?
Jimmy Kärnfeldt
December 16, 2025 AT 03:28Just wanted to say - this post made me feel less alone. I’ve been trying to explain this to my family for years, and they always say, ‘But patents are supposed to protect innovation!’ And I’m like… yeah, but not THIS kind of innovation. It’s like if you invented a car, then patented the color of the seatbelt buckle and sued everyone who tried to make a cheaper version. The system was meant to help people get new drugs fast - not lock them out for 15 years because a lawyer found a loophole in the pill’s shape.
Also, shoutout to the generic manufacturers who are fighting back. They’re the real heroes here.
Ariel Nichole
December 16, 2025 AT 13:45Love this. I work in health policy and this is exactly what we see on the ground. The 14-year cap sounds fair until you realize most drugs get approved with only 6-8 years left on the patent. So they go all-in on secondary patents - and guess what? The FDA doesn’t even evaluate whether those new versions are better. They just approve them. It’s not innovation - it’s paperwork magic. We need to stop rewarding companies for doing the bare minimum and start rewarding them for actual improvements. Otherwise, we’re just paying for the same pill in a fancier bottle.
Kaitlynn nail
December 17, 2025 AT 03:29