What exactly is an authorized generic?
An authorized generic is not a traditional generic drug. It’s the exact same medication as the brand-name version-same active ingredients, same manufacturing facility, same formula-but sold under a different label. Think of it like a car made in the same factory as a BMW, but sold under a different nameplate with no logos. The FDA defines it clearly: it’s a drug approved under the original New Drug Application (NDA), marketed by the brand company or its partner, with only packaging or labeling changes. You won’t find it listed in the FDA’s Orange Book because it doesn’t go through the Abbreviated New Drug Application (ANDA) process. That’s why it’s not just "similar" to the brand-it’s identical.
Why do authorized generics exist?
They were created to keep prices down during the 180-day exclusivity window granted to the first generic manufacturer after a brand drug’s patent expires. Instead of letting that first generic company monopolize the market, the brand company can launch its own generic version-called an authorized generic-to compete immediately. This drives prices lower faster. For example, when Prilosec lost patent protection in 2004, AstraZeneca launched an authorized generic within months. That move helped keep prices from spiking during the exclusivity period. Today, authorized generics make up about 3.2% of all generic drugs by volume, but they account for nearly 13% of generic drug revenue because they’re often used in higher-cost specialty medications.
How to spot an authorized generic by its packaging
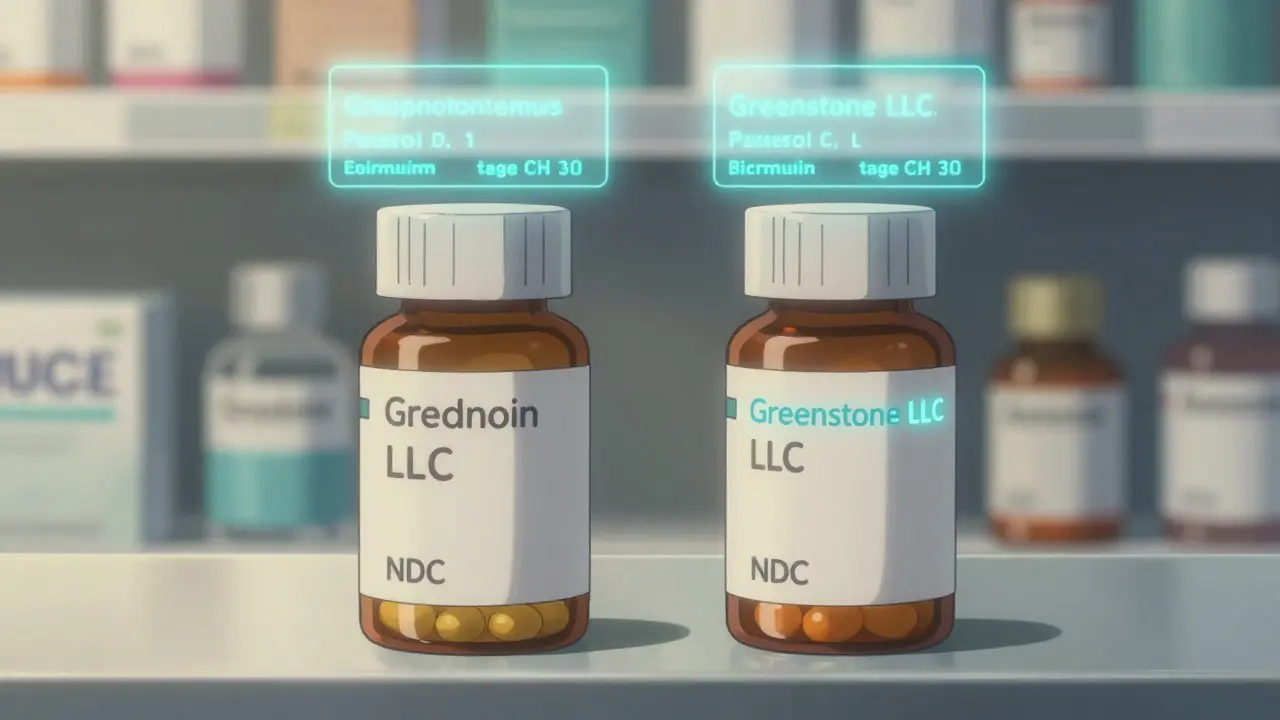
The easiest way to tell an authorized generic from the brand version is to look at the label. The brand name will be missing. Instead, you’ll see the name of a distributor like Greenstone LLC, Dr. Reddy’s, or Teva Pharmaceuticals. But here’s the key: the pill or capsule inside looks identical to the brand. No color change, no different shape-unless U.S. trademark laws force a minor visual tweak. That’s why pharmacists often get confused. The FDA doesn’t require any special "AG" or "Authorized Generic" marking on the box. So if you see "Distributed by [Company Name]" instead of "Manufactured by [Brand Name]," you’re likely looking at an authorized generic.
The NDC code is your best tool
The National Drug Code (NDC) is the only foolproof way to confirm an authorized generic. It’s a 10- or 11-digit number printed on the packaging. The NDC has three parts: labeler code, product code, and package code. For an authorized generic, the labeler code changes-it’s the distributor’s code, not the brand’s. But the product code and package code stay exactly the same as the brand version. For example, Protonix (pantoprazole) has a brand NDC starting with 00071. Its authorized generic, distributed by Dr. Reddy’s, has a labeler code of 55111-but the rest of the number matches perfectly. If you see a completely different product code, it’s a traditional generic. If the product and package codes match the brand, but the labeler code is different, it’s an authorized generic.

How to verify using the FDA’s official list
The FDA publishes a quarterly list of all active authorized generics. The most recent update was October 2, 2023, and it includes 147 products. You can find it on the FDA’s website under "Authorized Generic Drugs." This list tells you exactly which brand drugs have authorized versions, who distributes them, and what the correct NDCs are. Pharmacists use this daily. If you’re unsure about a medication, cross-check the NDC on the bottle with the FDA’s list. No other source is as reliable. Third-party databases like First Databank or Medi-Span are 98.7% accurate, but they pull their data from the FDA’s official list anyway.
Why authorized generics are often better than traditional generics
Traditional generics must prove they’re bioequivalent to the brand drug through testing. Authorized generics don’t need to-they’re made in the same factory, on the same machines, with the same ingredients. That means fewer batch-to-batch variations. Patients who switched from brand to traditional generic sometimes report differences in side effects or effectiveness. That’s rare with authorized generics. A 2022 Medscape survey found 92.6% of patients reported no difference in how they felt between the brand and the authorized generic. That’s because they’re the same drug, just in a different box.
Common mistakes people make when identifying them
Many assume that if the pill looks different, it’s not the same drug. But trademark laws prevent generics from copying the exact appearance, so even authorized generics might have slightly different markings. Others confuse them with "authorized brand" products-those are traditional generics sold in branded packaging, which are not the same. Another big mistake? Assuming the manufacturer name on the label is the drugmaker. It’s not. It’s the distributor. The real manufacturer is often the same as the brand’s. You can verify this by checking the FDA’s facility inspection reports. If the manufacturing site matches the brand’s, you’ve got an authorized generic.

What to do if you’re unsure at the pharmacy
If you pick up a prescription and notice the label looks different, ask the pharmacist: "Is this an authorized generic?" They can check the NDC against the FDA’s list in seconds. Don’t be afraid to ask. Pharmacists reported spending an average of 2.7 minutes per prescription verifying authorized generics in a 2022 NCPA survey. That’s because they’re trained to look for the right clues. If your pharmacy doesn’t have a system to flag them, request the NDC and look it up yourself on the FDA’s website. It’s free and takes less than a minute.
What’s changing in 2024?
The FDA plans to integrate authorized generic identifiers directly into the National Drug Code Directory by Q2 2024. That means pharmacy systems will automatically flag them, reducing errors. Right now, about 8.3% of generic drug dispensing errors are due to misidentifying authorized generics. That number should drop significantly once the new system rolls out. Also, the FTC is watching closely. Authorized generics typically cost 15-25% less than the brand but 5-15% more than traditional generics. That pricing sweet spot helps keep competition alive without undercutting innovation.
Final tip: Don’t trust appearance. Trust the code.
Don’t rely on color, shape, or brand name on the box. Those can change. The NDC code doesn’t. If the product and package codes match the brand, and the labeler code is different, you’ve got an authorized generic. And that’s a good thing. You’re getting the exact same medicine, often at a lower price, with no compromise in quality. The FDA, pharmacists, and patients all agree: authorized generics are the closest thing to the brand-without the brand price tag.
Are authorized generics the same as the brand-name drug?
Yes. Authorized generics are made in the same facility, using the same ingredients and manufacturing process as the brand-name drug. The only differences are the packaging, labeling, and distributor name. They are not bioequivalent-they are identical.
How do I know if my prescription is an authorized generic?
Check the NDC code on the bottle. If the product code and package code match the brand-name version, but the labeler code is different, it’s an authorized generic. You can verify this by searching the NDC on the FDA’s Authorized Generic Drug List.
Why does the manufacturer name on the label look different?
The brand-name company often partners with a subsidiary or another company to distribute the authorized generic. So instead of "Pfizer," you’ll see "Greenstone LLC" or "Teva Pharmaceuticals." But the actual manufacturing site is almost always the same as the brand’s.
Can authorized generics be cheaper than traditional generics?
Usually not. Authorized generics typically cost 5-15% more than traditional generics because they’re priced to compete with the brand, not undercut it. But they’re still 15-25% cheaper than the brand-name version. Their value is in consistency, not just price.
Do authorized generics have the same side effects as the brand?
Yes. Since they contain the exact same active and inactive ingredients, side effects are identical. Patients who switched from brand to authorized generic rarely report any change in how they feel.
Are authorized generics listed in the FDA’s Orange Book?
No. The Orange Book only lists drugs approved through the ANDA pathway. Authorized generics are marketed under the original NDA, so they don’t appear there. You must use the FDA’s separate Authorized Generic Drug List to find them.
Why do some authorized generics look different from the brand?
U.S. trademark laws prevent any drug from looking identical to a branded product, even if it’s made by the same company. So authorized generics may have a different color, shape, or imprint-but the active ingredient and dosage are unchanged.
Can I trust an authorized generic if I’ve had bad experiences with other generics?
Absolutely. Traditional generics must prove bioequivalence, which can sometimes lead to minor differences in how the drug is absorbed. Authorized generics don’t need to prove this-they’re the same drug, made the same way. If you’ve had issues with other generics, an authorized generic is often the best alternative.
Dayanara Villafuerte
January 17, 2026 AT 22:57kenneth pillet
January 19, 2026 AT 00:37Tyler Myers
January 20, 2026 AT 08:23Zoe Brooks
January 21, 2026 AT 13:52Kristin Dailey
January 22, 2026 AT 01:33Wendy Claughton
January 22, 2026 AT 11:46Aysha Siera
January 22, 2026 AT 19:55rachel bellet
January 23, 2026 AT 23:19Pat Dean
January 24, 2026 AT 15:21Selina Warren
January 26, 2026 AT 07:38