Drug shortages aren’t just inconvenient-they’re life-threatening. When a critical antibiotic or heart medication runs out, hospitals scramble. Patients delay treatment. Some die. And it’s not random. These shortages are predictable, preventable, and rooted in fragile supply chains that haven’t changed much since the 1990s. The truth? We’ve been relying on a system built for efficiency, not survival. Now, with global tensions, climate risks, and regulatory gaps, that system is breaking. But there’s a way out. Building resilient pharmaceutical supply chains isn’t about going back to the past. It’s about redesigning the future-step by step, with real tools, real data, and real strategy.
Why Your Medicine Might Not Be There When You Need It
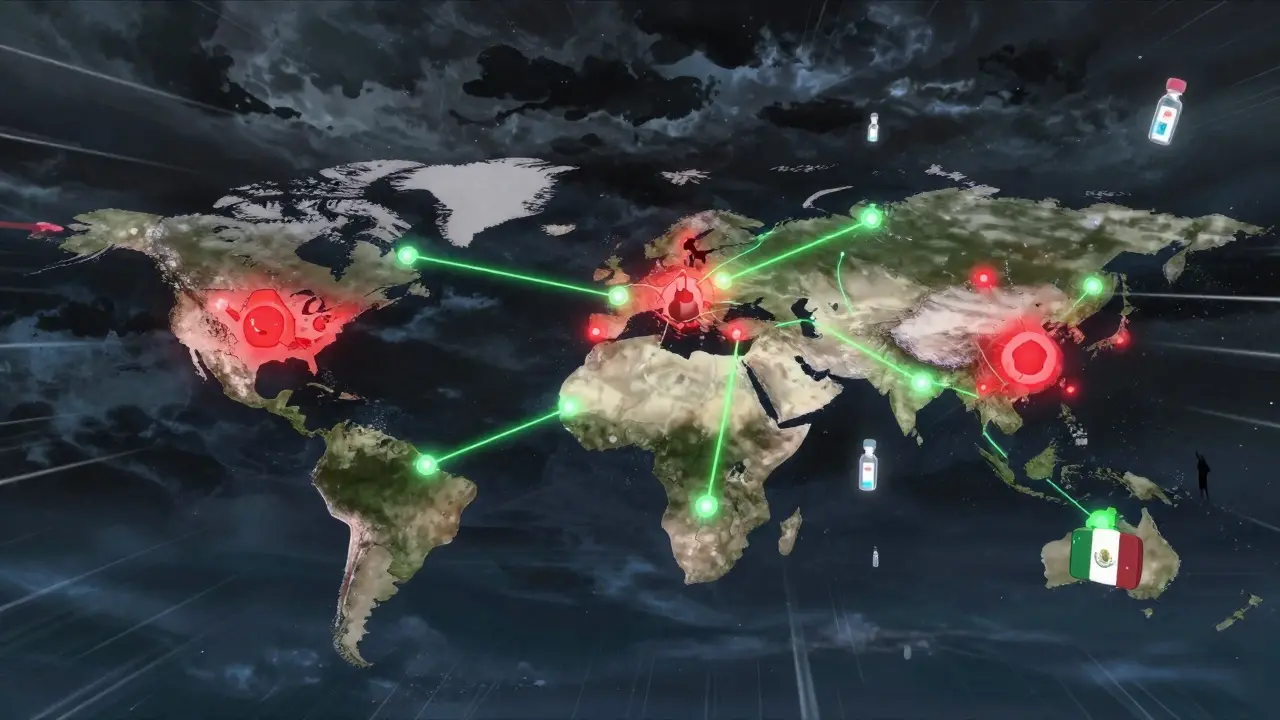
Most people don’t realize that 80% of the active ingredients in their pills come from just two countries: China and India. That’s not a coincidence-it’s a design choice. For decades, drugmakers chased lower costs, moving production overseas. The result? A single factory in a remote part of China can supply half the U.S. market for a life-saving drug. If that factory shuts down-due to a flood, a trade ban, or a labor strike-the entire supply chain freezes. In 2023, the FDA recorded over 300 drug shortages in the U.S. alone. Many were for injectables, antibiotics, and cancer drugs. These aren’t niche products. They’re essentials. The problem isn’t just foreign dependence. It’s concentration. A handful of suppliers control the entire global market for key APIs (active pharmaceutical ingredients). And those suppliers often rely on even fewer sources for their own raw materials. One breakdown at tier 5 or 6 of the supply chain can ripple up and cause a national shortage. This isn’t theory. It’s happened. During the pandemic, the U.S. ran out of generic injectable drugs because the Chinese supplier couldn’t get packaging materials from Vietnam. No one saw it coming because no one was mapping beyond tier 3.What Resilience Actually Means-And What It Doesn’t
Resilience doesn’t mean making everything in America. That’s a myth. The U.S. currently produces only 28% of the APIs it needs. Even if every domestic plant ran at full capacity, we still couldn’t replace the volume or variety we get from abroad. Trying to do so would raise drug prices by 20-30%, according to the National Academies of Sciences. That’s not a fix-it’s a trade-off with real human costs. True resilience is about balance. It’s having options. It’s knowing where your critical ingredients come from, how many suppliers you have, and what happens if one fails. The Mathematica Inc. report from 2023 defines it clearly: the ability to anticipate, prepare for, respond to, and recover from disruptions while keeping medicines flowing. That’s not magic. It’s logistics. And it’s doable.The Three Pillars of a Resilient Supply Chain
Every successful company building resilience uses the same three pillars: preparedness, response, and recovery. They’re not fancy. But they’re non-negotiable. Preparedness means knowing your risks. Leading firms now map 12 to 15 tiers of suppliers-not just their direct vendors, but their vendors’ vendors, and so on. They track geographic hotspots: 45% of API production is in China, 23% in India. They flag single points of failure. If a drug relies on one supplier in one city, it’s a red flag. They also build buffer stock. For essential medicines, top companies now keep 60 to 90 days of inventory on hand. That’s not waste-it’s insurance. The FDA recommends this for drugs with no alternatives. Response is about speed. When a disruption hits, you don’t have weeks to decide. You have hours. Companies that succeed have cross-functional teams ready to act. That means procurement, manufacturing, regulatory, and logistics all talk to each other daily-not once a quarter. One medtech firm reduced decision time by 50% during a disruption by creating a real-time dashboard that showed inventory levels, supplier status, and alternative sourcing options. No meetings. No emails. Just action. Recovery is about learning. After every disruption, companies ask: What broke? Why? How can we stop it next time? They don’t just fix the problem-they change the system. That’s how you avoid repeat failures.
Technology That’s Actually Making a Difference
You hear a lot about AI and blockchain in pharma. But most of it is hype. Here’s what’s working now:- Continuous manufacturing-Instead of making drugs in big batches over weeks, this method runs 24/7 in smaller, modular units. It cuts production time by 40%, reduces waste by 20%, and uses less space. One facility in North Carolina now makes a critical antiviral in 14 days instead of 90. The FDA has approved only 12 such systems so far, but that number is climbing fast.
- AI-powered risk forecasting-Tools now analyze weather patterns, political instability, shipping delays, and even social media chatter to predict disruptions 60 to 90 days ahead. One pilot program predicted a factory shutdown in India with 87% accuracy three months before it happened. That gave the company time to shift production.
- Blockchain traceability-It’s not just for crypto. In pilot programs, blockchain systems reduced counterfeit drugs by 75% by tracking every batch from raw material to pharmacy. If a batch is recalled, you know exactly where it went-and where it didn’t.
What Governments Are Doing-And What’s Still Missing
In 2025, the U.S. government launched the Strategic Active Pharmaceutical Ingredients Reserve. The goal? Stockpile 90 days’ worth of 150 essential medicines by 2027. That’s a start. But reserves alone won’t fix the system. They’re a safety net, not a solution. The CHIPS and Science Act allocated $1.2 billion to rebuild domestic manufacturing. That’s good. But it’s not enough. The industry needs $120 billion globally by 2030 to reach resilience targets. The government can’t pay for it all. But it can de-risk investment. That means faster approvals, tax credits for dual-sourcing, and shared infrastructure-like regional API hubs that multiple companies can use. Right now, the FDA takes 24 to 36 months to approve a new manufacturing process. For continuous manufacturing, they’ve cut that to 12 to 18 months. That’s progress. But regulators still treat every change like a new drug. They need to treat supply chain changes like maintenance-routine, expected, and safe.
How Companies Are Doing It-Real Examples
A large U.S. pharmaceutical company had a major shortage of a generic blood thinner in 2024. The supplier in China had a fire. The company had no backup. They lost $22 million in revenue and faced lawsuits. In 2025, they changed everything. They:- Identified 3 alternative API suppliers-one in Germany, one in South Korea, one in the U.S.
- Switched 80% of their sourcing to dual or triple suppliers.
- Invested in a $70 million modular manufacturing unit that can produce the drug in 12 months instead of 4 years.
- Created a real-time dashboard that alerts them if any supplier’s lead time increases by more than 10 days.
What You Can Do-Even If You’re Not a Pharma Giant
You don’t need $100 million to start building resilience. Here’s what any company can do today:- Map your top 5 critical drugs. Which ones would cause the most harm if they disappeared?
- Trace your suppliers. Who makes the API? Who makes the packaging? Who ships it? Go at least three tiers deep.
- Find one backup supplier. Even if it costs 10% more, get it now.
- Keep 30 days of buffer stock for your most critical items. Not 90. Just 30. That’s manageable.
- Ask your suppliers: What’s your disruption plan? If they don’t have one, find someone who does.
The Bottom Line: Resilience Costs Less Than Shortages
Building a resilient supply chain isn’t about spending more. It’s about spending smarter. Companies that invest in resilience avoid $14.7 million in losses per major disruption, according to ZS Associates. They also keep their customers alive. That’s not a cost center. That’s a mission. The old model-lean, global, just-in-time-was great when the world was stable. It’s not anymore. The next drug shortage isn’t a question of if. It’s when. The only question is: will you be ready?What causes most pharmaceutical supply chain disruptions?
The top causes are geopolitical tensions (like trade bans or war), natural disasters (floods, earthquakes), factory shutdowns due to regulatory issues or labor strikes, and single-source dependencies. Over 60% of disruptions trace back to a lack of supplier diversification, especially for active pharmaceutical ingredients (APIs) sourced from China and India.
Is domestic manufacturing the answer to drug shortages?
Not alone. While increasing U.S. production helps, trying to make everything domestically would raise drug prices by 20-30% without guaranteeing reliability. The real solution is a mix: strategic domestic capacity for critical drugs, plus diversified global sourcing. Relying on one country-even your own-creates new risks.
How much inventory should a hospital or pharmacy keep to prevent shortages?
For essential medicines with no alternatives, 60 to 90 days of buffer stock is recommended by the FDA and leading health systems. For other critical drugs, 30 days is a practical starting point. The key is prioritizing: focus on drugs used in emergencies, like antibiotics, insulin, and cardiac medications.
Can AI really predict drug shortages before they happen?
Yes. AI tools now analyze data from shipping logs, weather reports, political events, and supplier performance to forecast disruptions with 85-90% accuracy up to 90 days in advance. Early adopters have used this to reroute shipments, switch suppliers, or ramp up production before a crisis hits.
What’s the biggest mistake companies make when trying to build supply chain resilience?
Focusing only on cost. Many companies cut suppliers to save money, creating single points of failure. Resilience requires investing in redundancy-even if it costs more upfront. The real savings come from avoiding disruptions, not from trimming procurement budgets.
Are small pharmaceutical companies left behind in resilience efforts?
Not if they collaborate. While large firms spend 8-10% of their supply chain budget on resilience, small companies can join forces-sharing buffer stock, co-investing in modular manufacturing, or using shared logistics platforms. Partnerships are turning small players into resilient networks.